usp class vi testing
Why its important for silicon CVD coatings to be USP Class VI compliant. The standard implantation time required for a.
Dursan Passes Usp Class Vi Testing Why Is That Important
Testing to the highest ISO-10993 standards can add months of time and be very costly according to the Medical Device Testing Guide by Toxikon Inc.

. As our post on USP Class VI testing laid out biocompatibility is the measure of a materials lack of interaction with living tissue or a living system by not being toxic or. NES have been providing USP Class VI seals for many years and are considered industry experts in such products. AFT Fluorotec can manufacture a wide range of components using.
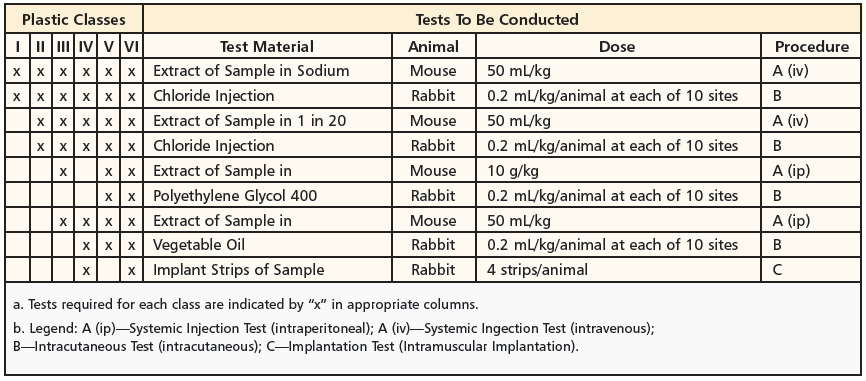
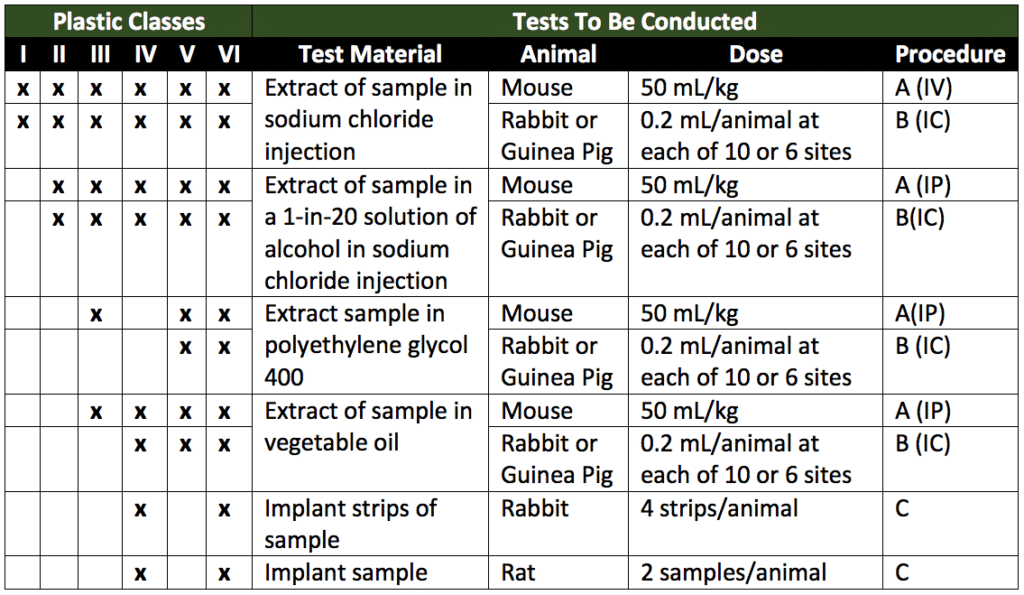
Class VI Test USP Project Number. Who uses USP Class VI elastomers. The Class Plastics tests.
In vivo testing USP. Both ISO 10993 and USP Class VI define testing requirements for biocompatibility the ability of a material to perform a desired function without causing adverse effects on the. Originally developed to test.
Class Plastics Testing USP USP Class Plastic Tests are designed to assess the biological reactivity of various types of plastics materials in vivo. Sanitary pumps require Class VI O-rings and seal material. How Dursan performed under USP Class VI test conditions.
AFT Fluorotec can manufacture a wide range of components using our USP Class VI PTFE compliant material. Tests are based on material extracts that. PBL provides of certificate of analysis for all.
On the day of the test the animals were. The implantation test determines the response of live tissue to the material when implanted into a live animal. United States Pharmacopeia USP sets standards for quality purity.
A plastic resin material that has passed Class VI certification is assumed to be more likely to produce favorable biocompatibility results. To test medical device biocompatibility manufacturers often use USP procedures such as the USP In Vivo Biological Reactivity Tests Class I-VI Plastics Tests. About USP Class VI.
Sanitary diaphragm valves have USP Class VI diaphragms. Two rabbits were used for the Implantation Test. In particular regarding the USP class VI certification process materials have to pass the biological tests ie.
Watershed 11122XC 723 Implant Test.

Usp Class Vi Certification Presco Marking Products And Engineered Films
Silicone Expanding The Horizon For Modern Medical Devices Medical Design And Outsourcing
Biopharmaceutical Usp Class Vi Gaskets Newman Sanitary Gasket

Fda And Usp Class Vi O Rings Guide 2020 Nes
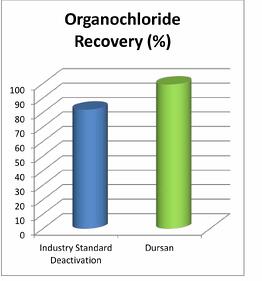
Dursan Passes Usp Class Vi Testing Why Is That Important
Ethide Laboratories What Is Systemic Injection Cytotoxicity Testing For Medical Devices And Drugs

Usp Class Vi Foster Corporation

Understanding Food Grade Vs Biocompatibility For Medical Device Materials Medical Product Outsourcing
Usp Class Vi Plastic Sheet For Medical Device Applications

O Rings Fda And Usp Class Vi Darcoid Rubber Company Oakland California

The Big Three Cytotoxicity Sensitization Irritation Testing Youtube

The Value Of Usp Class Vi Testing For Medical Device Cable And Wire Medical Design Briefs

What Is Usp Class Vi Testing Tbl Plastics

Optimum Class Vi Dispensing Components Nordson Efd

Usp Class Vi Foster Corporation

Compliance Of Teflon Encapsulated O Rings By M Cor Inc

Duraform Pa Certification Usp Class Vi Iso 10993 And Food Contact

Usp Class Vi Zulassung Was Bedeutet Das Reichelt Chemietechnik Magazin
